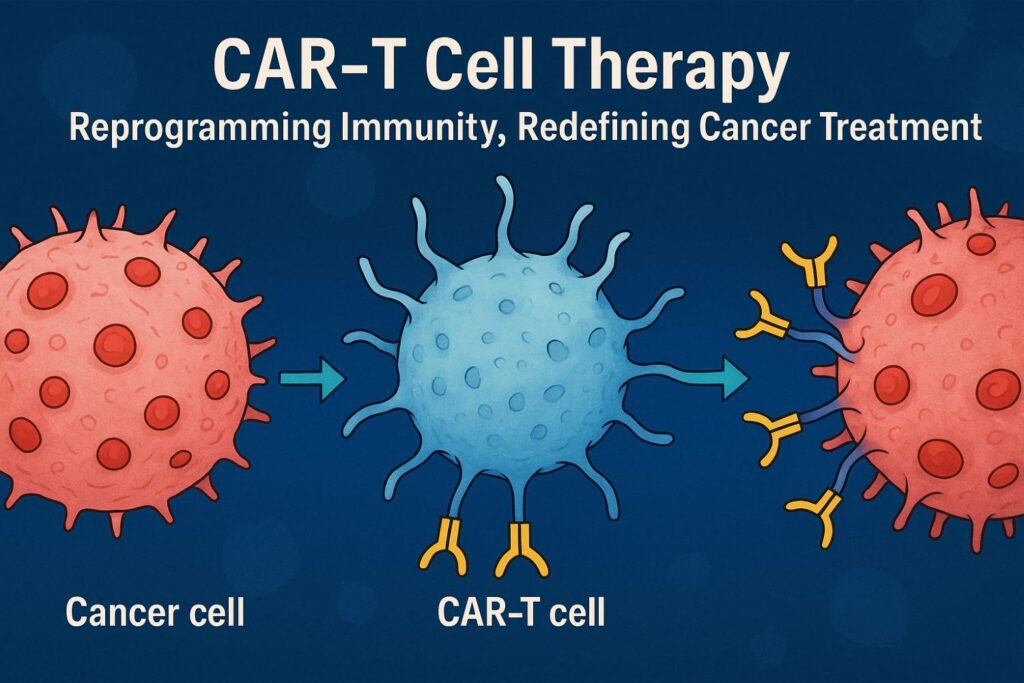
Chimeric antigen receptor (CAR)-T cell therapy has revolutionized cancer immunotherapy in the 21st century, providing innovative solutions and life-saving therapies for previously untreatable diseases. 1CAR-T cell therapy is a revolutionary new pillar in cancer treatment. CAR-T cell therapy has produced remarkably effective and durable clinical responses. CAR-T cell therapy received approval by the US Food and Drug Administration (FDA) in 2017.
CAR-T Cell therapy is a type of treatment in which a patient’s T cells (a type of immune system cell) are changed in the laboratory so they will attack cancer cells. T cells are taken from a patient’s blood. Then, the gene for a special receptor that binds to a certain protein on the patient’s cancer cells is added to the T cells in the laboratory. The special receptor is called a chimeric antigen receptor (CAR). Large numbers of the CAR-T cells are grown in the laboratory and given to the patient by infusion. CAR T-cell therapy is used to treat certain blood cancers, and it is being studied in the treatment of other types of cancer. Also called chimeric antigen receptor T-cell therapy.3
Major limitations of CAR-T cell therapy:
- life-threatening
- CAR-T cell-associated toxicities.
- limited efficacy against solid tumours
- inhibition and resistance in B cell malignancies
- Antigen escape
- Limited persistence
- Poor trafficking and tumour infiltration
- Immunosuppressive microenvironment
Many approaches including combining CAR-T cell therapy with other anticancer therapies or employing innovative CAR engineering strategies to improve anti-tumour efficacy, expand clinical efficacy, and limit toxicities have been proposed.
Ref 1. Patel K K. et.al., Molecular therapy Volume 33, Issue 5, 7 May 2025, Pages 2123-2140
Ref 2. Blood Cancer J. 11, 69 (2021). https://doi.org/10.1038/s41408-021-00459-7
Ref 3. https://www.cancer.gov/publications/dictionaries/cancer-terms/def/car-t-cell-therapy